Prof Pierre Emmanuel Rautou, Hepatology, Hôpital Beaujon; AP-HP, Inserm, Université Paris-Cité,
Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon. Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon.
Prof Pierre Emmanuel Rautou, Hepatology, Hôpital Beaujon; AP-HP, Inserm, Université Paris-Cité,
Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon. Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon.
Prof Pierre Emmanuel Rautou, Hepatology, Hôpital Beaujon; AP-HP, Inserm, Université Paris-Cité,
Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon. Global presentation of LIVER-TRACK project financed by the Agence Nationale de la Recherche (ANR) coordinated by Pr Pierre-Emmanuel Rautou, hepatologist at hôpital Beaujon.
Aims of LIVER-TRACK
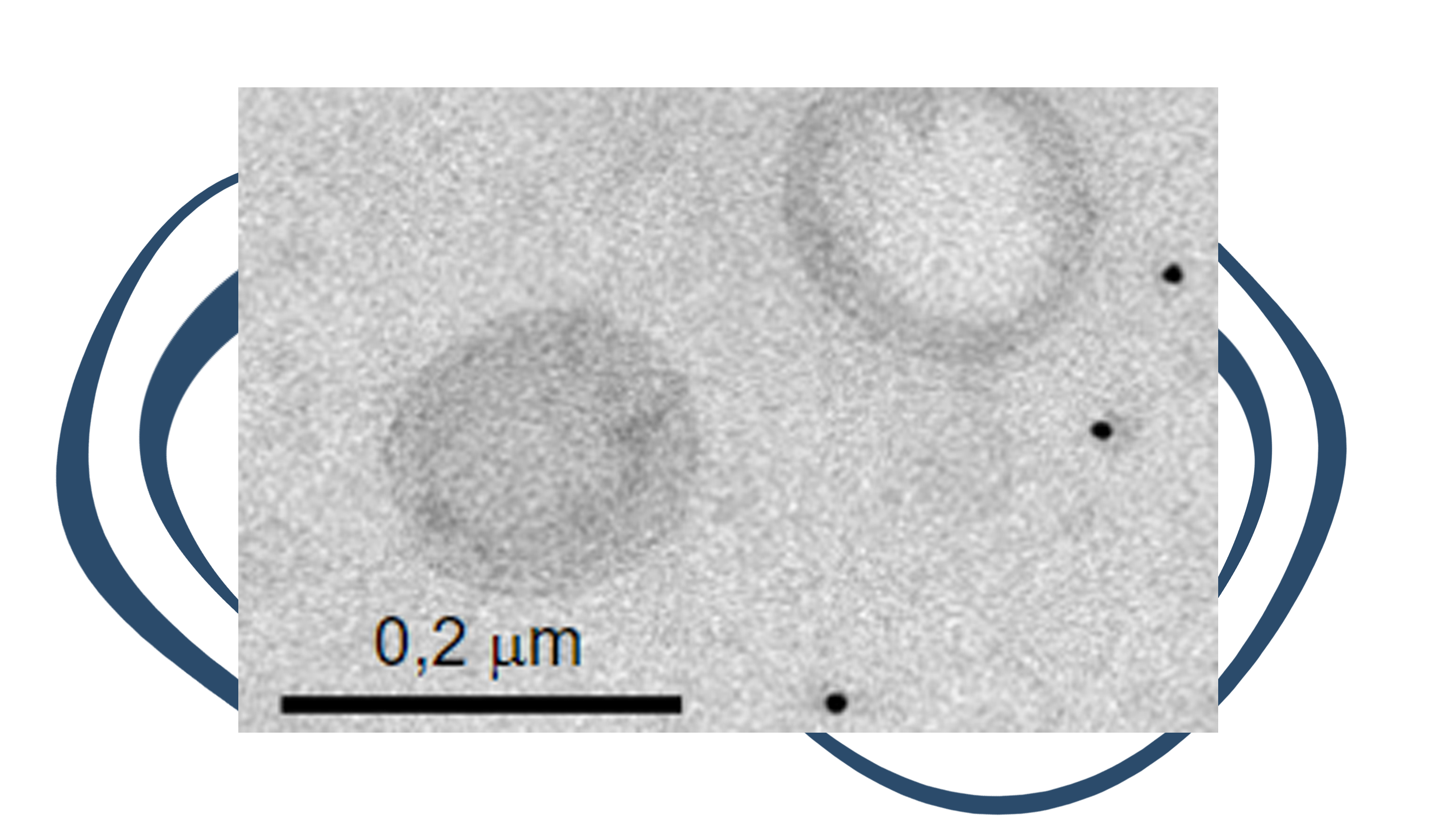
The objective of LIVER-TRACK is to address two unmet medical needs in patients with cirrhosis: the estimation of the risks of decompensation of cirrhosis, and of liver cancer development.
By bringing together the highly innovative measurements of circulating extracellular vesicles, an untapped source of biomarkers in liver diseases, with state-of-the-art existing biomarkers including FibroTest, cancer proteins and SNPs, LIVER-TRACK will create a Test for Decompensation and a Test for Liver Cancer.
These tests will be turnkey solutions to predict the evolution of patients with cirrhosis in routine clinical practice.
News
Assemblée Générale et SAB de LIVER-TRACK du 17 janvier 2025
LIVER-TRACK SAB members: Pr Annalisa Berzigotti, Pr Florence Gazeau and Pr Emmanouil Tsochatzis LIVER-TRACK members

LIVER-TRACK: Early Prediction of Cirrhosis Complications
LIVER-TRACK, one of 19 projects selected in the sixth wave of « hospital-university research » certification, is led by Inserm and coordinated by Professor Pierre-Emmanuel Rautou. Launched on May 23, 2024, and funded by the ANR under the France 2030 program, the project began on April 1, 2024, and will run for five years.
(Note: This article is in French.)
Aims of LIVER-TRACK
The objective of LIVER-TRACK is to address two unmet medical needs in patients with cirrhosis: the estimation of the risks of decompensation of cirrhosis, and of liver cancer development.
By bringing together the highly innovative measurements of circulating extracellular vesicles, an untapped source of biomarkers in liver diseases, with state-of-the-art existing biomarkers including FibroTest, cancer proteins and SNPs, LIVER-TRACK will create a Test for Decompensation and a Test for Liver Cancer.
These tests will be turnkey solutions to predict the evolution of patients with cirrhosis in routine clinical practice.
News
Assemblée Générale et SAB de LIVER-TRACK du 17 janvier 2025
LIVER-TRACK SAB members: Pr Annalisa Berzigotti, Pr Florence Gazeau and Pr Emmanouil Tsochatzis LIVER-TRACK members

LIVER-TRACK: Early Prediction of Cirrhosis Complications
LIVER-TRACK, one of 19 projects selected in the sixth wave of « hospital-university research » certification, is led by Inserm and coordinated by Professor Pierre-Emmanuel Rautou. Launched on May 23, 2024, and funded by the ANR under the France 2030 program, the project began on April 1, 2024, and will run for five years.
(Note: This article is in French.)
Project Timeline
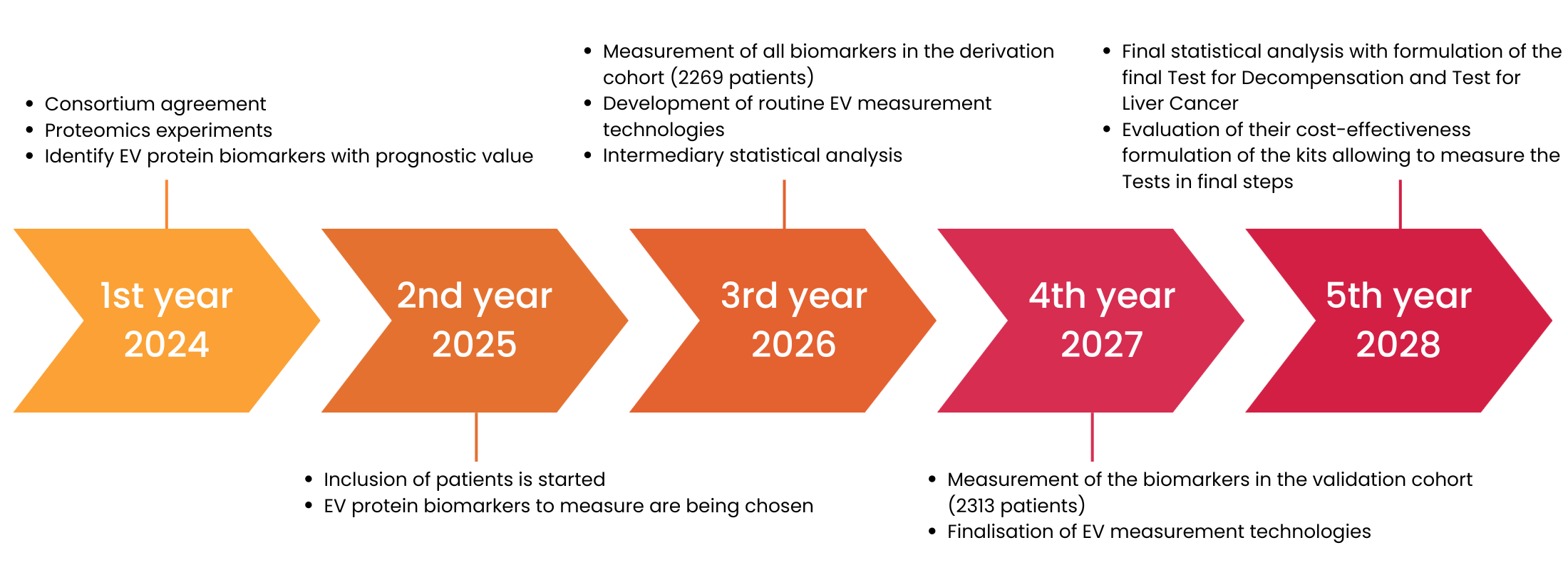
1st year 2024
- Consortium agreement
- Proteomics experiments
- Identify EV protein biomarkers with prognostic value
2nd year 2025
- Inclusion of patients is started
- EV protein biomarkers to measure are being chosen
3rd year 2026
- Measurement of all biomarkers in the derivation cohort (2269 patients)
- Development of routine EV measurement technologies
- Intermediary statistical analysis
5th year 2028
- Final statistical analysis with formulation of the final Test for Decompensation and Test for Liver Cancer
- Evaluation of their cost-effectiveness formulation of the kits allowing to measure the Tests in final steps